In one of the post where we figured out the library structure of the PIPseq$^{\textmd{TM}}$ V3 chemistry last year, we left a cliffhanger about how the PIPseeker$^{\textmd{TM}}$ software from FluentBio performed the barcode “translation”. During the translation, an original cell barcode that consists of four parts of “sub-barcodes” (8 bp + 6 bp + 6 bp + 8 bp) got mapped to a 16-bp barcode.
The V3 data
Using the species mixing data from the V3 chemistry mentioned in the previous post, we would end up with the following files in our working directory if we finished all procedures described in the previous post:
fluentbio
├── barcode
│ ├── barcoded_fastqs
│ │ ├── barcoded_1_R1.fastq.gz
│ │ ├── barcoded_1_R2.fastq.gz
│ │ ├── barcoded_2_R1.fastq.gz
│ │ ├── barcoded_2_R2.fastq.gz
│ │ ├── barcoded_3_R1.fastq.gz
│ │ ├── barcoded_3_R2.fastq.gz
│ │ ├── barcoded_4_R1.fastq.gz
│ │ └── barcoded_4_R2.fastq.gz
│ ├── barcodes
│ │ ├── barcode_whitelist.txt
│ │ └── generated_barcode_read_info_table.csv
│ ├── logs
│ │ ├── pipseeker_2023-05-25_16-53-40.log
│ │ └── progress.log
│ ├── metrics
│ │ └── barcode_stats.json
│ └── run_config.json
├── fastqs
│ ├── RK20220802_FR_3_S3_L001_R1_001.fastq.gz
│ ├── RK20220802_FR_3_S3_L001_R2_001.fastq.gz
│ ├── RK20220802_FR_3_S3_L002_R1_001.fastq.gz
│ └── RK20220802_FR_3_S3_L002_R2_001.fastq.gz
├── fb_v3_bc1.tsv
├── fb_v3_bc2.tsv
├── fb_v3_bc3.tsv
└── fb_v3_bc4.tsv
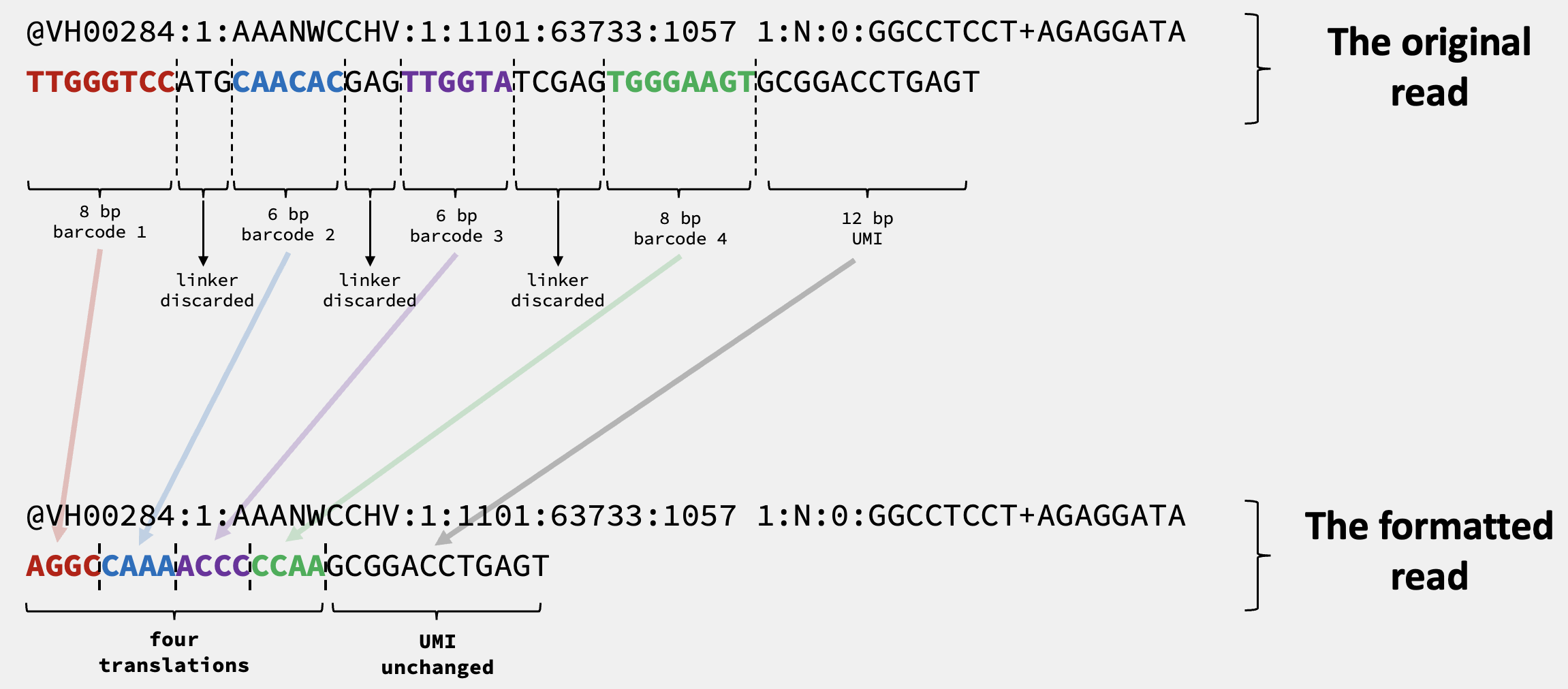
Again, using the read @VH00284:1:AAANWCCHV:1:1101:63733:1057 from the previous post as an example, we see that the read is like this in the original fastq file:
$ zcat fastqs/RK20220802_FR_3_S3_L001_R1_001.fastq.gz | grep -A 3 '@VH00284:1:AAANWCCHV:1:1101:63733:1057'
@VH00284:1:AAANWCCHV:1:1101:63733:1057 1:N:0:GGCCTCCT+AGAGGATA
TTGGGTCCATGCAACACGAGTTGGTATCGAGTGGGAAGTGCGGACCTGAGT
+
C;C!CCCCCCCCCCC-;CCCC;CCCCCCCCCCCCCCC!CCCCCCCCCCCCC
The same read is like this in the formatted fastq file by PIPseeker$^{\textmd{TM}}$:
$ zcat barcode/barcoded_fastqs/barcoded_1_R1.fastq.gz | grep -A 3 '@VH00284:1:AAANWCCHV:1:1101:63733:1057'
@VH00284:1:AAANWCCHV:1:1101:63733:1057 1:N:0:GGCCTCCT+AGAGGATA
AGGCCAAAACCCCCAAGCGGACCTGAGT
+
~~~~~~~~~~~~~~~~CCCCCCCCCCCC
Since now we know the library structure of the V3 chemistry should be:
[8-bp bc1]ATG[6-bp bc2]GAG[6-bp bc3]TCGAG[8-bp bc4][12-bp UMI]
There are 96 unique sequences of bc1, bc2, bc3 and bc4. The “translated” 16-bp cell barcodes in the formatted fastq file consists of four 4-bp barcodes, each with 96 unique sequences. Therefore, it is reasonable for us to assume the translation happens in a one-to-one manner, like shown below:
If we keep checking some reads, eventually we would figure out the dictionary for the translation. Let’s check all reads, comparing the original ones to their formatted counterparts, in a more systematic way. To this end, we first do the following processing using the command lines1:
join -1 1 -2 1 \
<(zcat barcode/barcoded_fastqs/barcoded_*_R1.fastq.gz | paste - - - - | cut -f 1,2 | sort -k1,1) \
<(zcat fastqs/RK20220802_FR_3_S3_L00*_R1_001.fastq.gz | paste - - - - | cut -f 1,2 | sort -k1,1) \
> mapping.txt
If you are not familiar with this type of bash command, here are some simple explanations. First, the
join -1 1 -2 1 file1 file2 > mapping.txt
part says that use the first column of file1 (-1 1) and the first column of file2 (-2 1) as the join keys. Merge the line from the two files side by side if the join keys are matched. Redirect the joined output to new text file called mapping.txt. Then file1 and file2 is essentially <(some commands), which means the join commands reads standard input, instead of actual files, from whatever comes out from the some commands in <(some commands). Finally, the paste - - - - in the parentheses output four lines from the original output into one line. In this case, each line after the paste command would be one read. The sort -k1,1 command basically sorts the read by read name (column 1), which is required by the join command.
Here is the top 10 lines from mapping.txt:
@VH00284:1:AAANWCCHV:1:1101:10013:11715 1:N:0:GGACTCCT+AGAGGATA AACAAGATCACCAGGCTATGTATTGCGT 1:N:0:GGACTCCT+AGAGGATA AAACTACAATGTTTCTCGAGGAAGAATCGAGCTCAAACATATGTATTGCGT
@VH00284:1:AAANWCCHV:1:1101:10013:12359 1:N:0:GGACTCCT+AGAGGATA ATCACCCCCAGTACAAGGTCATTTTGCA 1:N:0:GGACTCCT+AGAGGATA GTGAACTCATGCCAAATGAGATCAACTCGAGAGCATGCCGGTCATTTTGCA
@VH00284:1:AAANWCCHV:1:1101:10013:12548 1:N:0:GGACTCCT+AGAGGATA ACGTAAGAAGCTATCTGTTGATTCTCAA 1:N:0:GGACTCCT+AGAGGATA CCTATTTAATGTAGCGAGAGCTTGACTCGAGCAAGGTACGTTGATTCTCAA
@VH00284:1:AAANWCCHV:1:1101:10013:12965 1:N:0:GGACTCGT+AGAGGATA ACAGAGCGATTAACCGAGGCTGTATTCG 1:N:0:GGACTCGT+AGAGGATA CTGTTTCCATGGGTCTAGAGGAACAGTCGAGAGTAATGGAGGCTGTATTCG
@VH00284:1:AAANWCCHV:1:1101:10013:13154 1:N:0:GGACTCCT+AGAGGATA AAACAACAACAAACCTTAAGATAGGTAT 1:N:0:GGACTCCT+AGAGGATA CCTTTACAATGGGTTTCGAGTGGGTTTCGAGTCTAATTGTAAGATAGGTAT
@VH00284:1:AAANWCCHV:1:1101:10013:14555 1:N:0:GGACTCCT+AGAGGATA AATTAATCCACGCCTCAGCCCAGGTAGC 1:N:0:GGACTCCT+AGAGGATA CCACCTCTATGAAAGTGGAGACAAAGTCGAGACATGGACAGCCCAGGTAGC
@VH00284:1:AAANWCCHV:1:1101:10013:14858 1:N:0:GGACTCCT+AGAGGATA CCAGACAAAACGACCAGAATCAGCGCTG 1:N:0:GGACTCCT+AGAGGATA AATATGACATGAATAGCGAGGGTTTCTCGAGAATAAGGAGAATCAGCGCTG
@VH00284:1:AAANWCCHV:1:1101:10013:14934 1:N:0:GGACTCCT+AGAGGATA ATTACCTCCCTAAGCTCTACCGCTGCAT 1:N:0:GGACTCCT+AGAGGATA AAAGAGGCATGGCTCTTGAGATCTTCTCGAGGGTTAGGGCTACCGCTGCAT
@VH00284:1:AAANWCCHV:1:1101:10013:15312 1:N:0:CGACTCCT+AGAGGATA CCCCAGTGAATCCCCGACAGTTGGTGAG 1:N:0:CGACTCCT+AGAGGATA CTAACGCCATGTAACCCGAGTAGAACTCGAGCAAGGGTTACAGTTGGTGAG
@VH00284:1:AAANWCCHV:1:1101:10013:16070 1:N:0:GGACTCCT+AGAGGATA CAAGCAGGCAGGATTACAAGGTAGGTTG 1:N:0:GGACTCCT+AGAGGATA CCATCCACATGGCTAAGGAGGCACTATCGAGTTTGCCAGCAAGGTAGGTTG
As you can see, we paired the formatted reads with the original ones side-by-side. Now we could compare the formatted and original sequences within the pair. That is, the 3rd and the 5th columns (space-delimited). We could write a python script to do this. Note that everything is done under the fluentbio working directory:
from collections import defaultdict
wl1 = [line.strip() for line in open('./fb_v3_bc1.tsv')]
wl2 = [line.strip() for line in open('./fb_v3_bc2.tsv')]
wl3 = [line.strip() for line in open('./fb_v3_bc3.tsv')]
wl4 = [line.strip() for line in open('./fb_v3_bc4.tsv')]
translate1 = {}
translate2 = {}
translate3 = {}
translate4 = {}
for w,t in zip([wl1, wl2, wl3, wl4], [translate1, translate2, translate3, translate4]):
for i in w:
t[i] = defaultdict(int)
with open('mapping.txt') as fh:
for line in fh:
_, _, bcout, _, bcin = line.strip().split(' ') # we only care about the 3rd and 5th columns
key1 = bcin[:8]
key2 = bcin[11:17]
key3 = bcin[20:26]
key4 = bcin[31:39]
out1 = bcout[0:4]
out2 = bcout[4:8]
out3 = bcout[8:12]
out4 = bcout[12:16]
if key1 in wl1: # only record when key1 is in the white list of bc1
translate1[key1][out1] += 1
if key2 in wl2: # only record when key2 is in the white list of bc2
translate2[key2][out2] += 1
if key3 in wl3: # only record when key3 is in the white list of bc3
translate3[key3][out3] += 1
if key4 in wl4: # only record when key4 is in the white list of bc4
translate4[key4][out4] += 1
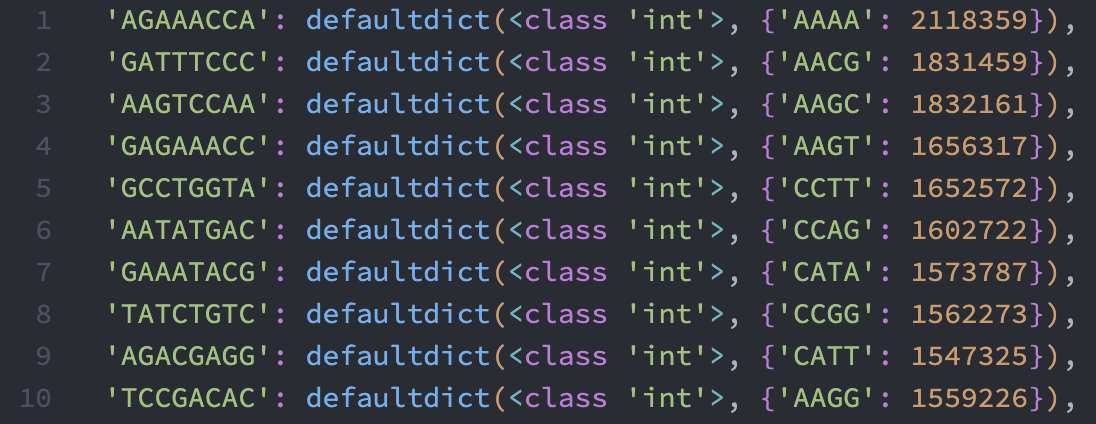
The four dictionaries translate{1..4} contain the PIPseeker$^{\textmd{TM}}$. If the software is indeed using a one-to-one translation, we should expect in translate{1..4} that each original barcode in the whitelist should only have one entry or one dominating entry. It turned out that this is the case. Here is a screenshot of the first 10 entries in translate1:
I have now organised all four translations into csv files, and you can download them from the scg_lib_structs GitHub repo.
The V4 data
Since we know that the V4 chemistry uses the same whitelist as the V3 chemistry, there are just some variable bases at the beginning of Read 1 to desync the Illumina sequencing cycles2. Therefore, it is reasonable for use to assume that the translation would remain the same. Is that the case? Let’s find out.
First, we downloaded the T20 V4 Mouse Brain Nuclei data from FluentBio. We used the exact same procedures to get the mapping.txt file. Then a slightly different python script was needed to figure out the translation. Due to the variable bases at the beginning of Read 1, we used the built-in regular expression module re. The content of the script looks like this:
import re
from collections import defaultdict
# compile the pattern with groups
pattern = re.compile(r'([ACGTN]{8})ATG([ACGTN]{6})GAG([ACGTN]{6})TCGAG([ACGTN]{8})')
wl1 = [line.strip() for line in open('../fb_v3_bc1.tsv')]
wl2 = [line.strip() for line in open('../fb_v3_bc2.tsv')]
wl3 = [line.strip() for line in open('../fb_v3_bc3.tsv')]
wl4 = [line.strip() for line in open('../fb_v3_bc4.tsv')]
translate1 = {}
translate2 = {}
translate3 = {}
translate4 = {}
for w,t in zip([wl1, wl2, wl3, wl4], [translate1, translate2, translate3, translate4]):
for i in w:
t[i] = defaultdict(int)
with open('mapping.txt') as fh:
for line in fh:
_, _, bcout, _, bcin = line.strip().split(' ')
res = pattern.search(bcin)
if not (res is None): # only look for matching records
key1, key2, key3, key4 = res.groups()
out1 = bcout[0:4]
out2 = bcout[4:8]
out3 = bcout[8:12]
out4 = bcout[12:16]
if key1 in wl1:
translate1[key1][out1] += 1
if key2 in wl2:
translate2[key2][out2] += 1
if key3 in wl3:
translate3[key3][out3] += 1
if key4 in wl4:
translate4[key4][out4] += 1
Here is a screenshot of the first 10 entries in translate1:
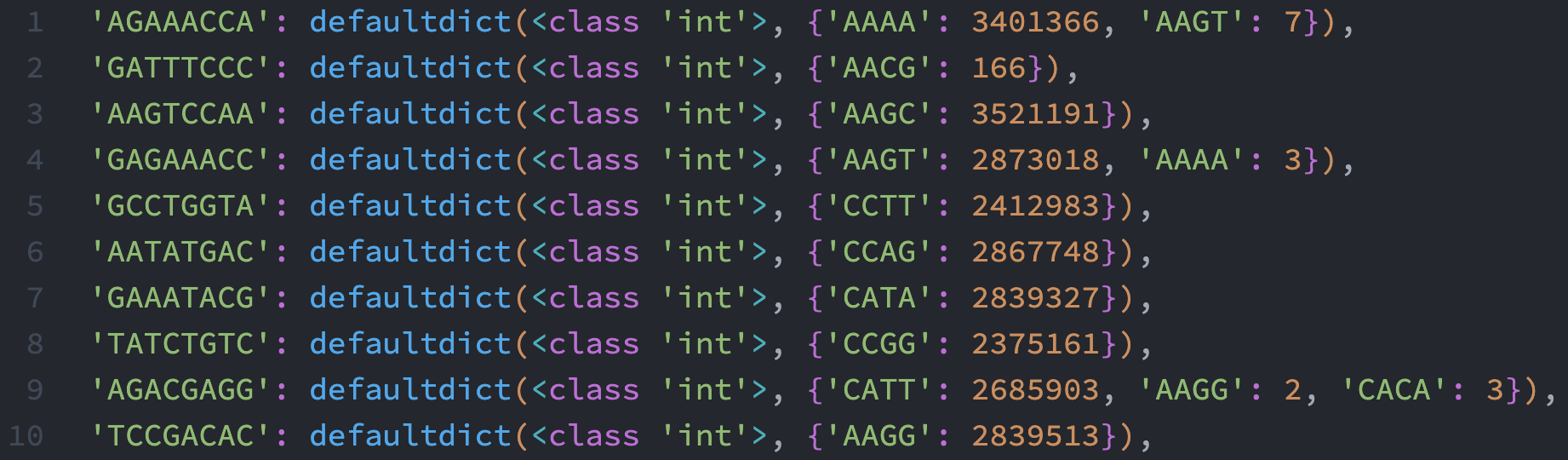
As you can see that this data have some noise there. However, each entry in the original whitelist only have one dominating 4-mer after the translation. The top dominating one is orders of magnitude higher than the rest, so I guess it is safe to just use the 4-mer with the highest count.
Eventually, it turns out that the translation remains unchanged between the V3 and V4 chemistries.
Not sure if this the most efficient way of achieving this purpose. If you have better ideas, please let me know. ↩︎
Check the previous post where I talked about the V4 library structure. ↩︎